Introduction:
Have you ever wondered which atmospheric layer contains the vital shield that protects us from the sun’s harmful ultraviolet rays? The answer lies in the stratosphere and ozone layer, a critical duo ensuring life can thrive on Earth.
The stratosphere and ozone layer play a fundamental role in safeguarding our planet. Located roughly 10 to 30 miles above the Earth’s surface, the stratosphere is home to the ozone layer, which absorbs and scatters ultraviolet solar radiation.
This protective barrier is essential for preventing skin cancers, cataracts, and other health issues caused by UV exposure. Understanding the importance of the stratosphere and ozone layer helps us appreciate their role in maintaining the balance of our climate and ecosystems.
This article will explore the stratosphere and ozone layer, as well as their composition, functions, and challenges due to human activities and environmental changes.
Structure of the Atmosphere: Different Layers
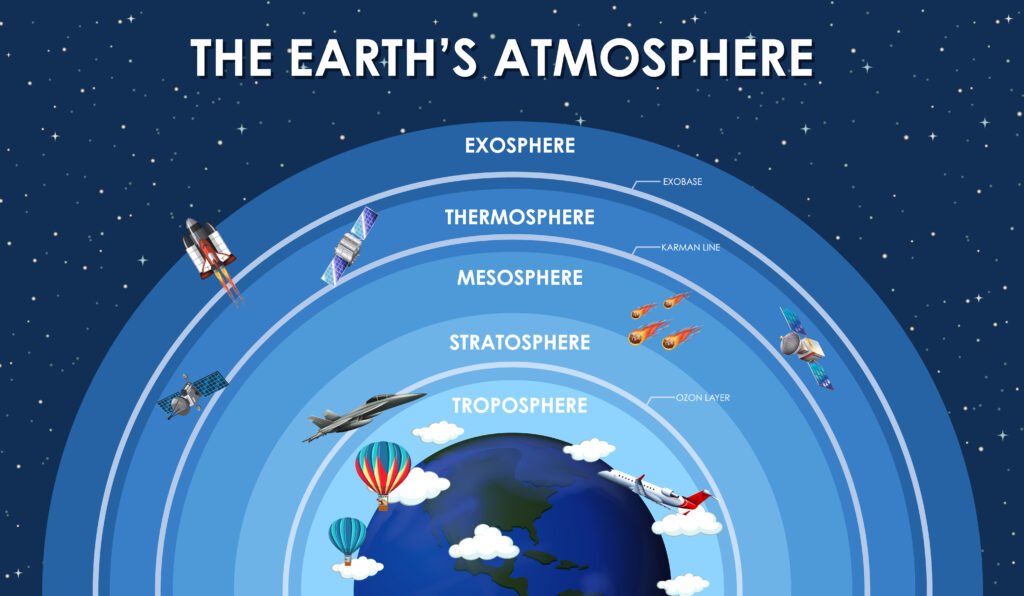
- Troposphere: This is the lowest part of the atmosphere, where weather phenomena such as clouds, rain, snow, etc., occur. The temperature rises 6.5 degrees Celsius per 1km of ascent in this layer.
- Stratosphere: It extends upwards from the tropopause up to 50 km. It consists of much of the ozone in the atmosphere. This increases the temperature and height because of the taking in of ultraviolet rays from the sun.
- Mesosphere: The region over the stratosphere is the mesosphere. Here, the temperature again decreases to about -90 degrees Celsius.
- Thermosphere: This layer lies over the mesopause and is where temperature rises. This rise is due to solar radiation that breaks down the nucleus into molecules and atoms. The region is above 100 km and is known as the ionosphere. It is the hottest layer of the atmosphere.
- Exosphere: This region is about 500 km and is referred to as the ionosphere, which contains oxygen and hydrogen atoms and follows ballistic trajectories under the influence of gravity.
Composition and Function of the Ozone Layer:
Ozone is a molecule produced by three oxygen atoms and is often called O3. It is created under heat and sunlight, which catalyzes the chemical reactions between nitrogen oxides (NOx) and volatile organic compounds (VOC), which are considered hydrocarbons.
How the Ozone Layer Protects Life on Earth:
The ozone layer has a higher level of ozone that protects the earth from the sun’s harmful ultraviolet radiation. The ozone layer absorbs 97-99% of the harmful radiations from the sun, which can decline life on Earth; otherwise, millions of people would have skin diseases and weak immune systems.
Ozone Depletion:
The protective shield of ozone has been threatened for a long time. Different anthropogenic activities have triggered the layer’s thinning, causing the development of an Ozone Hole. Faulty human actions, environmental degradation, pollution, and other factors are vital to ozone depletion.
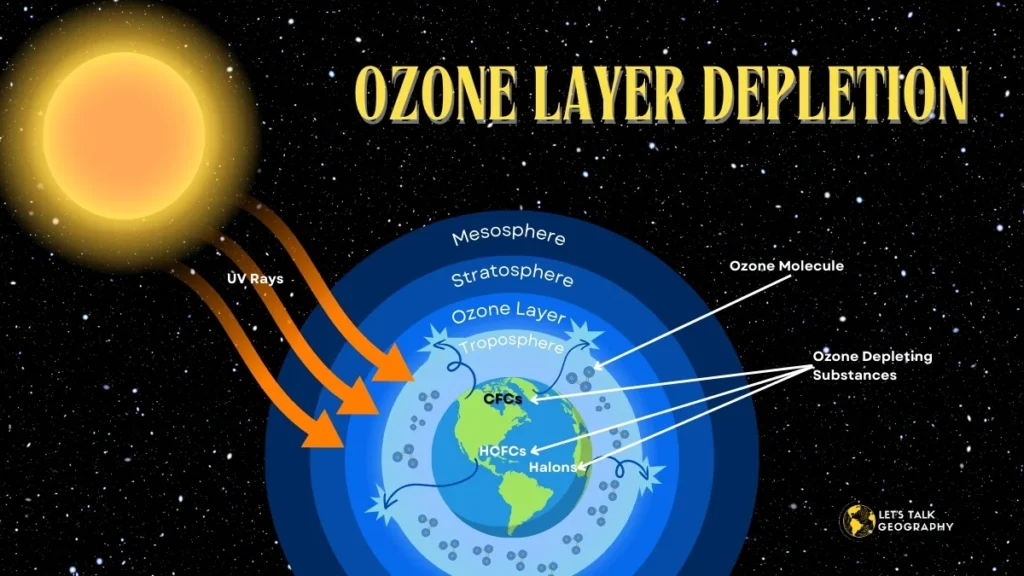
◆ Causes of Ozone Depletion:
- Chlorofluorocarbons (CFCs): Chlorofluorocarbons can break down chlorine, fluorine, and carbon atoms, which are extremely stable. This huge amount of stability makes CFCs slowly reach the stratosphere. Chlorine can degrade the ozone layer as it acts as a catalyst. Chlorine initiates the breakdown of ozone and gets mixed up with the freed ozone to create oxygen molecules; as the ozone molecules get broken, they lose their ability to absorb radiation, affecting the earth.
- Other Ozone-Depleting Substances: Ozone-depleting substances other than CFCs include HCFCs, halons, methyl bromide, carbon tetrachloride, and methyl chloroform. ODS are mainly very influential in the troposphere and only decline under the maximum ultraviolet light in the stratosphere.
- Natural Factors Affecting the Ozone Layer: While human activities are the primary cause of ozone depletion, natural factors like volcanic eruptions and solar cycles can also influence the ozone layer. Volcanic eruptions release sulfur dioxide and other gases into the atmosphere, which can contribute to ozone depletion. Solar cycles, which cause variations in the sun’s UV radiation, can also affect the ozone layer. However, these natural factors have a much smaller impact than human-caused ozone depletion.
◆ Effects of Ozone Depletion:
- Increased UV Radiation: Due to the depletion of the ozone layer, increased ultraviolet rays adversely affect humans. This might result in hazardous human health issues, which include skin diseases, cancer, cataracts, quick aging, and a weak immune system. When the stratospheric ozone layer declines, higher levels of UVB reach the Earth’s Surface.
- Health Impacts on Humans: A critical public concern is related to the effects of increased surface UV radiation on human health. So, human health damage is evident. The ozone hole is growing over Antarctica and a huge part of Australia, New Zealand, Chile, and Argentina. It causes skin burns, skin cancer, and cataracts. It might cause basal and squamous cell carcinomas directly linked to UV-B exposure.
- Environmental Consequences: Animals are being affected by depletion in the ozone layer, widespread epidermal damage, which is damaging crops, and increased formation of cyanobacteria, which are influenced by UV radiation. This is causing excessive damage to plant DNA. Plant damage leads to shoot damage with biomass and leaf area, which changes the height of plants. It catalyzes the production of harmful volatile organic compounds, such as isoprenes.
The Ozone Hole:
An ozone hole is a layer or region formed by exceptionally depleted ozone in the stratosphere. It is a place in the atmosphere with ozone values lower than 220 DU.
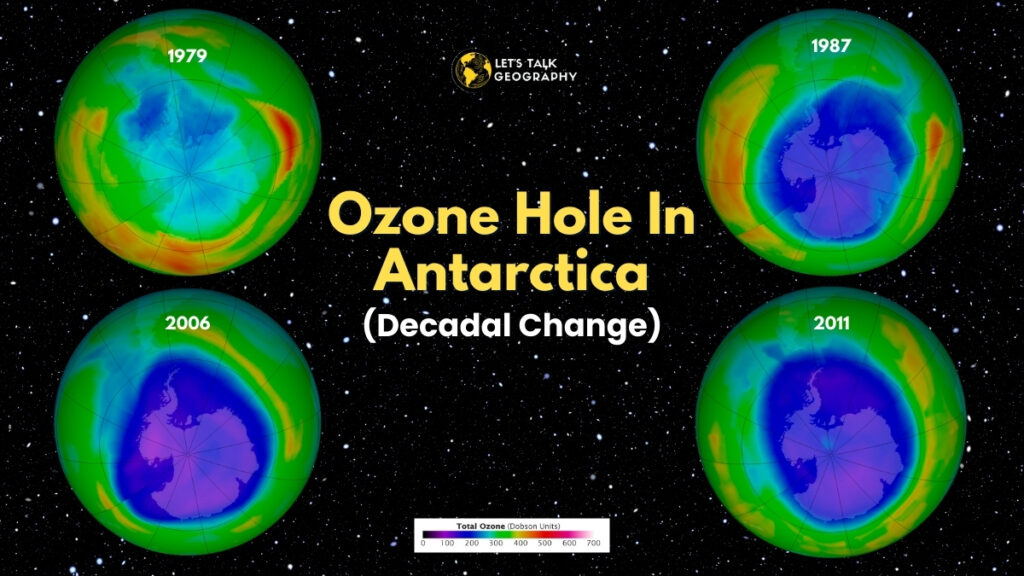
◆ Discovery and Development:
The introduction of the Antarctic ozone hole was initially declared in a paper by British Antarctic Surveys by Joe Farman. Ozone Hole is calculated by Dobson, a spectrophotometer designed in 1920.
◆ Geographic Regions Affected:
Countries that are affected by ozone depletion are mostly Antarctica because the ozone hole is growing and making it hazardous for Australia, New Zealand, Argentina, and Chile.
Prevention of Ozone Depletion:
◆ Signing the Montreal Protocol:
The Montreal Protocol, an international treaty signed in 1987, has been instrumental in phasing out ozone-depleting substances. This landmark agreement demonstrates that positive change is possible when nations unite to address environmental challenges. The ozone layer’s recovery is a success story, but it’s still unfolding. Continued monitoring, research, and adherence to the Montreal Protocol are essential to ensure the long-term health of the ozone layer.
As a result of the Montreal Protocol, the concentration of ODS in the atmosphere has begun to decline, and the ozone layer is slowly recovering. However, full recovery is expected to take several decades.
◆ Promoting the use of ozone-friendly alternatives:
HFCs (hydrofluorocarbons) have replaced CFCs in many applications, but they are potent greenhouse gases, and their use is being phased down under the Kigali Amendment to the Montreal Protocol. Choose ozone-friendly products. Look for appliances and products that are labeled as “ozone-friendly” or “CFC-free.”
◆ Others:
- Raising awareness: Educating the public about the importance of the ozone layer and the actions they can take to protect it.
- Supporting research: Ongoing research is essential to monitor the ozone layer’s recovery and identify emerging threats.
- Properly dispose of old appliances: Ensure that old refrigerators and air conditioners are disposed of correctly to prevent the release of ODS.
- Reduce energy consumption: Conserving energy indirectly reduces the demand for power generation, which can release ODS.
Conclusion:
In conclusion, understanding the stratosphere and ozone layer is crucial for appreciating their role in protecting life on Earth. The stratosphere, located 10 to 30 miles above the surface, houses the ozone layer, which absorbs harmful ultraviolet radiation. Decades ago, the discovery of ozone depletion, caused primarily by human-made chemicals, spurred global concern and action. Through concerted efforts, we’ve made significant progress in restoring this vital layer of the atmosphere.
While the ozone layer’s recovery is a reason for optimism, it’s crucial to remember that our planet faces other pressing environmental issues, including climate change. The lessons learned from protecting the ozone layer can guide us in addressing these new challenges. By working together, investing in scientific research, and making informed decisions, we can continue to safeguard Earth’s delicate systems for future generations.
Call to action:
To protect our vital stratosphere and the ozone layer, join the global effort to reduce ozone-depleting substances. Stay informed, support environmental policies, and choose eco-friendly products. Your actions make a difference in preserving our planet’s protective shield. Learn more and take action today.
References:
- Layers of the atmosphere. (n.d.). Niwa. Co.Nz. Retrieved June 3, 2024, from https://niwa.co.nz/atmosphere/layers-atmosphere
- How is ozone formed? (n.d.). Scdhec.gov. Retrieved June 3, 2024, from https://scdhec.gov/environment/your-air/most-common-air-pollutants/about-ozone/how-ozone-formed
- Does the ozone layer protect the earth from? (2022, July 4). Byjus.com; BYJU’S. https://byjus.com/question-answer/the-ozone-layer-protects-the-earth-from/
- Us Epa, O. (2017). Basic ozone layer science. https://www.epa.gov/ozone-layer-protection/basic-ozone-layer-science
- Ozone-depleting substance. (2017, February 14). European Environment Agency. https://www.eea.europa.eu/help/glossary/eea-glossary/ozone-depleting-substance
- The ozone hole. (2022, June 30). British Antarctic Survey. https://www.bas.ac.uk/data/our-data/publication/the-ozone-layer/
What is the ozone layer, and where is it located?
The ozone layer contains high ozone concentration (O3) molecules in the Earth’s stratosphere. It is located approximately 10 to 30 miles above the Earth’s surface. The ozone layer is crucial in absorbing and scattering ultraviolet (UV) radiation from the sun, protecting living organisms from harmful UV rays that can cause skin cancer, cataracts, and other health issues.
Why is the ozone layer important?
The ozone layer is required for life on Earth because it absorbs most of the sun’s harmful ultraviolet (UV) radiation. Without the ozone layer, these UV rays would reach the Earth’s surface in higher intensities, leading to increased skin cancer, cataracts, and other health problems. Additionally, excessive UV radiation can harm marine ecosystems, decrease crop yields, and disrupt the balance of various ecological systems.
How does the stratosphere contribute to the ozone layer’s function?
The stratosphere, where the ozone layer is situated, provides a stable environment that allows ozone molecules to form and remain concentrated. The stratosphere’s relatively calm and stratified air prevents the rapid mixing in the lower atmospheric layers, which helps maintain the ozone layer’s integrity. The unique conditions in the stratosphere, including the presence of ultraviolet light, facilitate the continuous formation and breakdown of ozone, sustaining its protective role.
What causes ozone layer depletion?
Ozone layer depletion is mainly caused by human-made chemicals called ozone-depleting substances (ODS), such as chlorofluorocarbons (CFCs), halons, and other similar compounds. These items release chlorine and bromine atoms in the stratosphere, which then react with ozone molecules, leading to their breakdown. This process significantly reduces the ozone concentration in the stratosphere, resulting in the thinning of the ozone layer and the making of the ozone hole.
What is the ozone hole, and where is it most prominent?
The ozone hole is a region of significantly reduced ozone concentration in the stratosphere, primarily observed over Antarctica. It forms annually during the Southern Hemisphere’s spring (September to November) due to ozone-depleting substances and the unique meteorological conditions in the polar stratosphere. The ozone hole has also been observed over the Arctic to a lesser extent. Monitoring and addressing the ozone hole is critical for protecting the ozone layer and mitigating its impacts on global health and the environment.
How can we protect the ozone layer?
Protecting the ozone layer requires global cooperation to decline and eliminate the use of ozone-depleting substances (ODS). International agreements, including the Montreal Protocol, have successfully phased out many ODS and promoted safer alternatives. Individuals can also give by using products that do not contain ODS, supporting policies and initiatives aimed at ozone protection, and staying informed about environmental issues. Continued research, regulation, and public awareness are essential for the ongoing recovery and preservation of the ozone layer.

