Introduction:
In the intricate tapestry of life on Earth, biogeochemical cycles play a pivotal role, acting as the backbone of the ecosystem‘s sustainability. These cycles – carbon, nitrogen, phosphorus, and water – are nature’s way of recycling essential elements, ensuring their continuous availability for all living organisms.
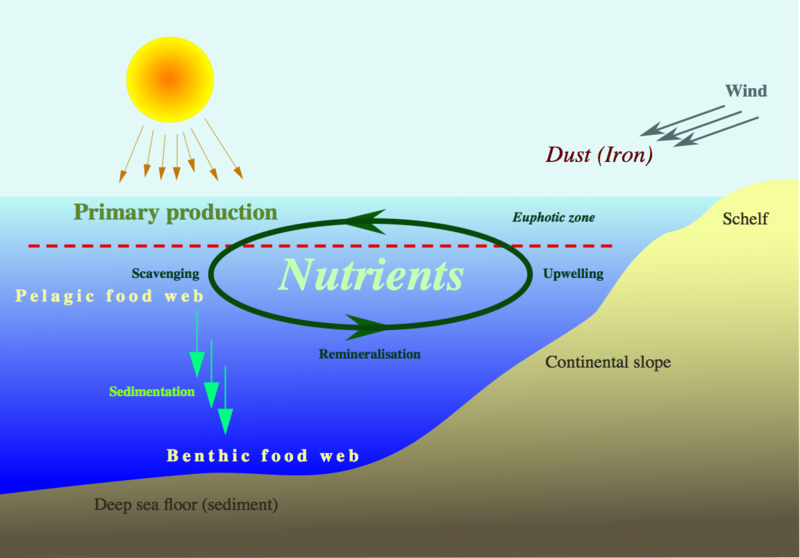
Human activities have a profound impact on these delicate natural processes. Understanding these cycles is not just an academic pursuit; it is crucial for maintaining the delicate balance of life on our planet and for shaping a sustainable future.
What are Biogeochemical Cycles?
A biogeochemical cycle refers to the movement and exchange of chemical elements among the biological (living organisms), geological (the physical Earth), and chemical (involving chemical processes) components of the ecosystem. These cycles are pathways by which essential elements like carbon, nitrogen, phosphorus, and water are cycled through the ecosystem, ensuring their availability for use by living organisms.
In a biogeochemical cycle, an element will move through various forms and locations, such as the atmosphere, hydrosphere (water bodies), lithosphere (Earth’s crust), and biosphere (living organisms). For example, in the carbon cycle, carbon dioxide is taken up by plants during photosynthesis and converted into organic matter, which then moves through the food chain. Eventually, it is released back into the atmosphere through respiration, decay, or combustion.
Why are Biogeochemical Cycles Important?
These cycles are critical for life on Earth as they regulate the availability of essential elements necessary for various life processes. They also play a key role in maintaining the Earth’s climate and other environmental conditions. Disruptions to these cycles, often caused by human activities, can lead to significant environmental and ecological consequences.
The importance of biogeochemical cycles can be highlighted through the following points:
Supporting Life: Biogeochemical cycles provide essential elements necessary for living organisms to build proteins, DNA, and other biological molecules. Without these cycles, life as we know it would not be sustainable.
Regulating Climate: Cycles like the carbon cycle play a crucial role in regulating Earth’s climate. For example, the carbon cycle controls the levels of carbon dioxide, a key greenhouse gas, in the atmosphere, thus influencing global temperatures.
Maintaining Ecosystem Health: These cycles ensure that nutrients are continually recycled in ecosystems, maintaining soil fertility and the health of aquatic systems, which is vital for the survival of various plant and animal species.
Balancing Geological Processes: Elements like phosphorus and nitrogen, which are crucial for life, are circulated from the Earth’s crust to living organisms and back, demonstrating the interconnection between biological and geological processes.
Water Purification: The water cycle includes natural filtration processes, such as percolation through the soil, which helps in purifying water, making it safe for consumption and use by living organisms.
Agricultural Productivity: The nitrogen and phosphorus cycles are particularly important for agriculture. These cycles replenish the soil with nutrients essential for crop growth, thus supporting food production.
Waste Decomposition: Biogeochemical cycles involve the breakdown of waste materials, which is a crucial process for recycling nutrients and preventing the accumulation of harmful substances in the environment.
Supporting Biodiversity: By ensuring a consistent supply of essential nutrients in various forms, these cycles support a wide range of habitats and thus contribute to biodiversity.
Indicating Environmental Changes: Changes in biogeochemical cycles can serve as indicators of environmental stress or change, such as increased carbon dioxide levels indicating global warming.
Human Health: Elements like carbon, nitrogen, and phosphorus, cycled through these processes, are essential for human health. Disruptions in these cycles can lead to health issues, such as nutrient deficiencies or exposure to harmful substances
What are the Key Biogeochemical Cycles of the Ecosystem?
There are several major biogeochemical cycles, each involving multiple processes that include biological, geological, and chemical interactions.
The Carbon Cycle:
Carbon is a fundamental component of organic life. The carbon cycle involves the movement of carbon between the atmosphere, oceans, terrestrial biosphere, and geological formations. Photosynthesis by plants captures carbon dioxide from the atmosphere, converting it into organic matter. This carbon is then transferred through the food chain. Respiration, decomposition, and combustion release it back into the atmosphere or soil, completing the cycle.
The various stages and processes involved in the carbon cycle are
Photosynthesis: This is the starting point of the carbon cycle. Plants, algae, and certain bacteria capture carbon dioxide (CO2) from the atmosphere. Through the process of photosynthesis, these organisms use sunlight to convert CO2 and water into glucose (a simple sugar) and oxygen. The glucose is used to build more complex organic molecules like cellulose, which makes up the structure of the plants.
Carbon in the Food Chain: The carbon incorporated into plants becomes part of their structure. When animals consume plants, they assimilate this carbon into their bodies for energy and growth. As the carbon moves up the food chain, from herbivores to carnivores, it is continuously transferred between organisms.
Respiration: Both plants and animals release CO2 back into the atmosphere through respiration. This process is the reverse of photosynthesis, where organisms use oxygen to break down glucose, releasing energy, water, and CO2.
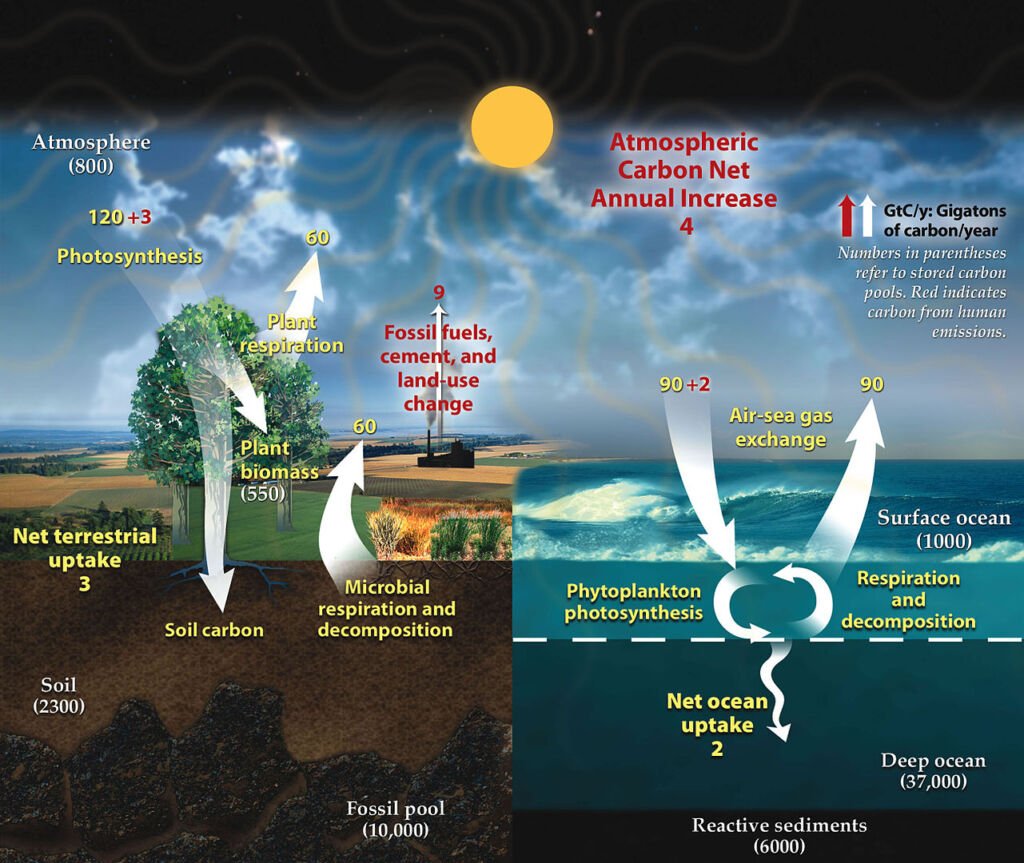
Decomposition: When plants and animals die, decomposers like bacteria and fungi break down their bodies. During this decomposition process, the organic carbon is converted back into CO2, which is released into the atmosphere or soil.
Combustion: The burning of fossil fuels (like coal, oil, and natural gas), which are ancient deposits of organic material, releases a significant amount of CO2 into the atmosphere. Similarly, forest fires convert the carbon stored in trees into CO2.
Carbon in Oceans: The oceans also play a crucial role in the carbon cycle. CO2 from the atmosphere dissolves in the surface waters of the ocean. Some of this carbon is taken up by marine organisms, like plankton, through photosynthesis. Additionally, some dissolved CO2 reacts with seawater to form carbonate compounds, which can be used by marine organisms to make shells.
Geological Carbon Storage: Over long time scales, some carbon is stored in geological formations. For example, dead marine organisms with carbonate shells sink to the ocean floor, contributing to sediment layers. Over millions of years, these sediments can turn into rock, trapping the carbon. Additionally, organic material can be buried and, under high pressure and temperature, transformed into fossil fuels, storing the carbon underground.
The carbon cycle is a fine balance of these processes, maintaining the levels of CO2 in the atmosphere, which is crucial for life on Earth. However, human activities, particularly the burning of fossil fuels and deforestation, are disrupting this balance, leading to an increase in atmospheric CO2 and contributing to climate change.
The Nitrogen Cycle:
Nitrogen is crucial for all living organisms as it is a key component of amino acids and nucleic acids. The nitrogen cycle starts with nitrogen fixation, where atmospheric nitrogen is converted into ammonia by bacteria. This ammonia is then used by plants or converted into nitrates and nitrites. After consumption and assimilation by organisms, nitrogen returns to the soil through waste and decomposition. Denitrification returns nitrogen to the atmosphere.
The cycle involves several steps:
Nitrogen Fixation: This is the first step where atmospheric nitrogen (N2), which makes up about 78% of the Earth’s atmosphere, is converted into a form that can be used by living organisms. This conversion is primarily done by certain types of bacteria, known as nitrogen-fixing bacteria. These bacteria, some of which live in the soil and others in symbiotic relationships with plants (like legumes), convert atmospheric nitrogen into ammonia (NH3).
Nitrification: Once ammonia is introduced into the soil, other types of bacteria convert it into nitrites (NO2-) and then into nitrates (NO3-). Nitrates are the form of nitrogen most easily used by plants. This process is called nitrification and is performed by two groups of bacteria: Nitrosomonas, which converts ammonia to nitrite, and Nitrobacter, which converts nitrite to nitrate.
Assimilation: Plants absorb nitrates and ammonia from the soil through their roots. Once absorbed, these compounds are used to synthesize amino acids, nucleic acids, and other nitrogen-containing organic compounds. When animals eat these plants (or other animals), they assimilate the nitrogen into their own bodies for the same purposes.
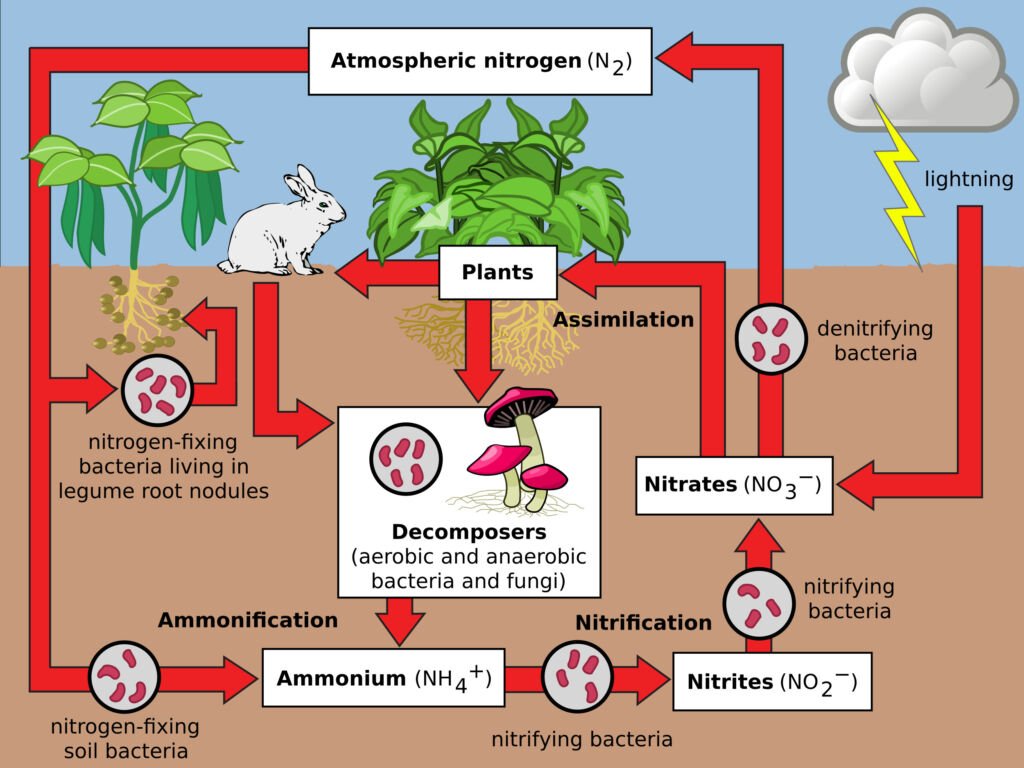
Ammonification: After the death of an organism, or through waste products like urine and feces, the nitrogen in the organic matter is returned to the soil. Decomposers like bacteria and fungi break down this organic matter, releasing nitrogen back into the soil as ammonia. This process is known as ammonification.
Denitrification: This is the final step in the nitrogen cycle. Denitrification is carried out by a different set of bacteria that convert nitrates back into nitrogen gas (N2), which is then released back into the atmosphere. This process is important as it helps maintain the balance of nitrogen in the atmosphere.
The nitrogen cycle is a closed loop that ensures the continuous availability of nitrogen in a form that can be utilized by living organisms. However, human activities such as the use of nitrogen-based fertilizers, burning fossil fuels, and deforestation are impacting this cycle, often leading to environmental issues like eutrophication and the greenhouse effect.
The Phosphorus Cycle:
Phosphorus is vital for the formation of DNA, cell membranes, and bones. Unlike carbon and nitrogen, phosphorus lacks a gaseous phase. The cycle begins with the weathering of rocks that release phosphate into the soil and water. Plants absorb phosphate, which then passes through the food chain. It returns to the soil through waste and decomposition. Over time, sedimentation can turn back into rock, starting the cycle anew.
The key steps in this cycle are
Weathering of Rocks: The cycle begins with the weathering of rocks. Over time, rocks that contain phosphorus minerals break down due to various environmental factors like rain, wind, and temperature changes. This weathering process releases phosphate ions (PO4^3-) into the soil and water bodies.
Absorption by Plants: Plants absorb phosphate ions from the soil through their roots. Phosphorus is a crucial element for plants, required for several vital functions, including the formation of DNA, ATP (adenosine triphosphate, a molecule used for energy transfer in cells), and cell membranes.
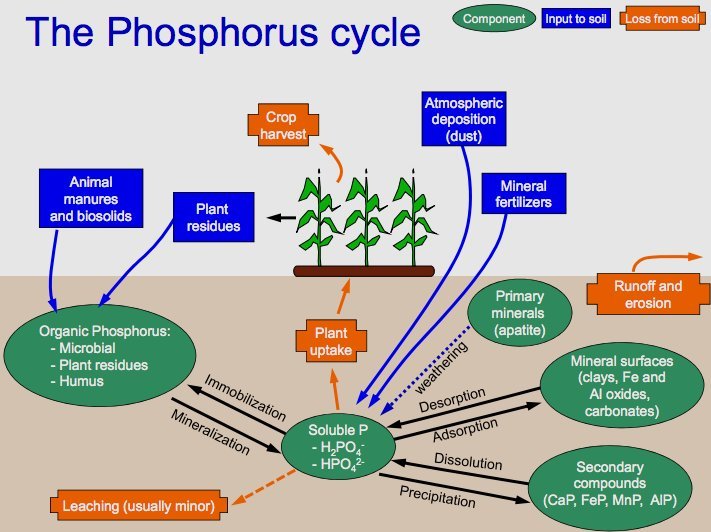
Movement Through the Food Chain: As animals consume plants, the phosphorus contained within the plant matter is transferred into their bodies. It is used for various biological processes, such as the formation of bones and teeth in animals. Phosphorus moves up the food chain as these animals are consumed by other predators.
Return to the Soil: When plants and animals die, their bodies undergo decomposition, a process facilitated by microbes in the soil. During decomposition, the organic form of phosphorus is converted back into inorganic phosphate. Animal waste also contributes to returning phosphorus to the soil.
Sedimentation and Geological Formation: Some phosphate ions can be washed away from the soil into rivers and eventually into the oceans. In water bodies, these phosphates can be incorporated into marine organisms or settle on the ocean floor. Over long periods, the accumulation of these sediments can lead to the formation of new phosphorus-rich rock layers through geological processes.
Uplift and Weathering: Geological uplift can bring these deep, phosphorus-containing rock layers back to the surface. The cycle comes full circle when these rocks are again subjected to weathering, releasing phosphate back into the soil, and the cycle repeats.
The phosphorus cycle is a slow-moving cycle, especially compared to the carbon and nitrogen cycles. Human activities, such as mining phosphate rocks for fertilizers and detergents, and agricultural runoff, can significantly alter this cycle, leading to environmental issues like eutrophication in aquatic systems.
The Water Cycle:
Water is essential for life, and its cycle involves evaporation, transpiration, condensation, precipitation, and runoff. Water evaporates from surfaces and transpires from plants, condensing into clouds in the atmosphere. It then precipitates back to the surface as rain or snow and eventually flows back into oceans or groundwater, continuing the cycle.
Different stages in the water cycle are
Evaporation: This is the process by which water changes from a liquid to a gas or vapor. Water from various sources, such as oceans, rivers, lakes, and even soil moisture, absorbs heat from the sun and evaporates into the atmosphere. This is a primary method by which water enters the atmospheric part of the water cycle.
Transpiration: Plants also contribute to the water cycle through transpiration. This process involves the release of water vapor from plant leaves into the atmosphere. Transpiration occurs as plants absorb water from the soil and transport it to their leaves, where some of it is released as vapor through small pores called stomata.
Condensation: As water vapor rises higher into the atmosphere, it cools and condenses into tiny water droplets or ice crystals, forming clouds. This condensation process is aided by the presence of small particles in the air, such as dust or sea salt, which serve as nuclei for the formation of droplets.
Precipitation: When these water droplets or ice crystals in the clouds become too heavy to remain suspended in the air, they fall to the Earth’s surface as precipitation. This can occur in various forms, including rain, snow, sleet, or hail, depending on the atmospheric conditions.
Runoff and Infiltration: Once precipitation reaches the ground, it follows two main paths. It can flow over the land surface as runoff, eventually reaching rivers, lakes, and oceans. Alternatively, some of the water may infiltrate (seep) into the soil, replenishing groundwater stores. Groundwater can then move through the ground and resurface in springs, or it may flow into rivers and oceans.
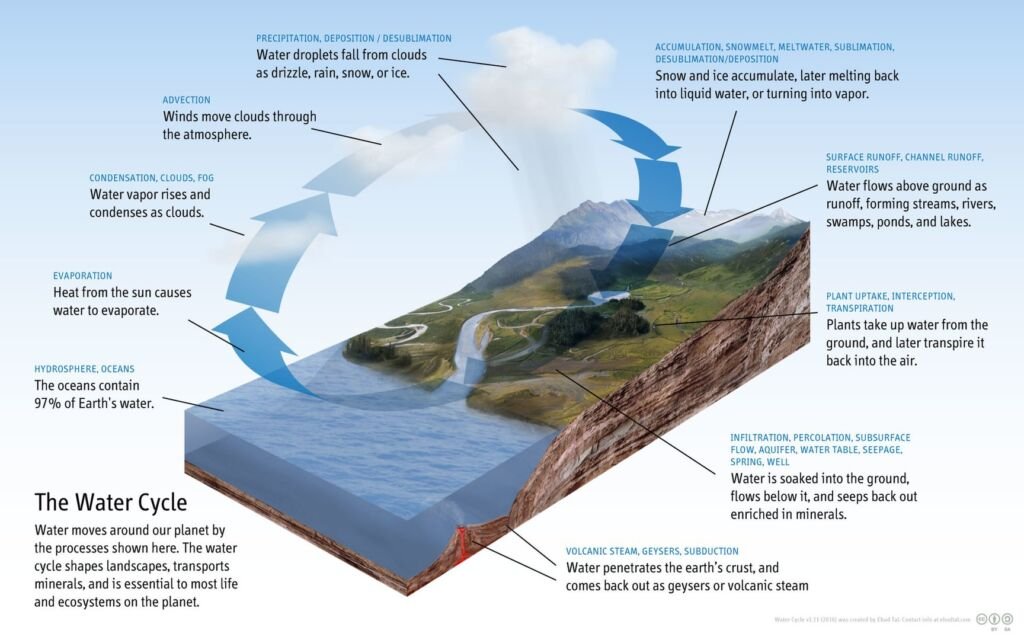
Collection: Water collected in bodies of water like rivers, lakes, and oceans will eventually evaporate back into the atmosphere, restarting the cycle. Additionally, groundwater contributes to this process by feeding into these water bodies.
The water cycle is a crucial component of Earth’s climate system and plays a key role in redistributing heat through atmospheric and oceanic circulation. It is also essential for maintaining ecosystems, as it affects patterns of vegetation, weather, and climate. Human activities, such as water extraction, deforestation, and urbanization, can significantly impact the natural flow of the water cycle, leading to environmental challenges like droughts, floods, and changes in climate patterns.
Sulpher Cycle:
The sulfur cycle is a complex biogeochemical cycle that involves the movement of sulfur through the biosphere, atmosphere, hydrosphere, and lithosphere. Sulfur is an essential element for life, playing a critical role in the structure and function of proteins and enzymes. The sulfur cycle differs from other nutrient cycles in that it includes both atmospheric and terrestrial components and involves a variety of chemical forms of sulfur.
The cycle involves several steps:
Mineralization and Dissimilation: When plants, animals, and microbes die, their sulfur-containing compounds are decomposed by microorganisms. This process, known as mineralization, converts these compounds into inorganic forms, such as hydrogen sulfide (H2S), sulfate ions (SO4^2-), and elemental sulfur (S).
Assimilation: Plants absorb sulfate ions from the soil and convert them into organic sulfur compounds, which are essential for proteins and vitamins. Animals obtain sulfur by consuming plants or other animals.
Emission to the Atmosphere: Sulfur can be released into the atmosphere in several ways. Volcanic eruptions emit sulfur dioxide (SO2) and other sulfur compounds. In wetlands, bacteria can convert sulfate in the soil to hydrogen sulfide gas (H2S), which is then released into the atmosphere. Additionally, the burning of fossil fuels and industrial processes release significant amounts of SO2 into the atmosphere.
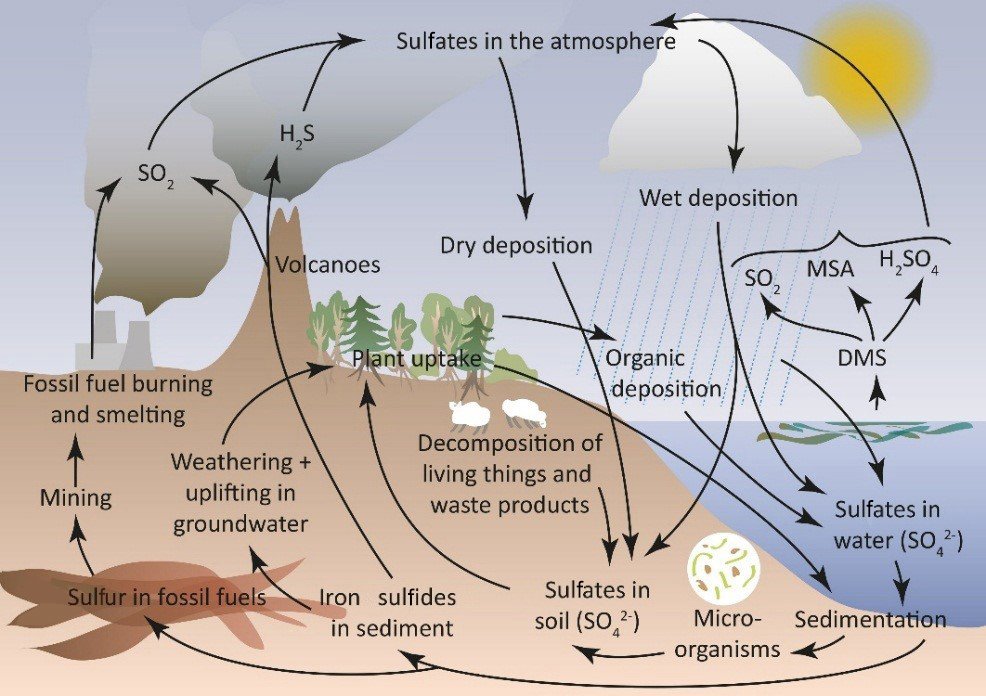
Atmospheric Processes: In the atmosphere, sulfur compounds can undergo a variety of chemical reactions. SO2 can react with oxygen to form sulfate aerosols, or it can dissolve in water droplets to form sulfurous acid, contributing to acid rain
Deposition: Sulfur compounds in the atmosphere can return to the Earth’s surface through wet deposition (rain, snow) or dry deposition (aerosols, gases). This deposition is a major way in which sulfur is returned to the Earth, where it can re-enter terrestrial and aquatic ecosystems.
Leaching and Runoff: Sulfates in the soil can be leached by rainwater and carried to rivers and oceans. In water bodies, these sulfates can be assimilated by aquatic plants and microorganisms or remain in the water.
Sedimentation and Burial: Over geological timescales, sulfur compounds can become trapped in sedimentary rock formations. Through tectonic activity and weathering, these sulfur compounds can be released back into the sulfur cycle.
The sulfur cycle is intricately linked with climate regulation and ecosystem health. However, human activities such as industrial processes, fossil fuel combustion, and agriculture have significantly altered the sulfur cycle, leading to environmental issues such as acid rain, air pollution, and the disruption of aquatic and terrestrial ecosystems. Understanding and managing human impacts on the sulfur cycle is crucial for maintaining the health of our environment.
