Rare earth elements have existed since the formation of the Earth, although their existence was not discovered until the late 18th century. Because of their molecular similarities, the history of specific rare-earth elements is both complex and confusing.
There were also reports of the finding of a huge number of other “elements” that were intended to be rare-earth members but were not.

Many of the “newly found elements” were combinations of up to six separate REEs. Theoreticians were able to establish that only 14 lanthanides exist because of Bohr’s hydrogen atom theory, which was published in 1913–14.
The existence of 13 of these elements was confirmed by Moseley’s experiments. Scientists devoted their entire lives seeking to obtain a 99% pure rare earth over the 160 years of discovery (1787–1947).
Scientists discovered element 61 in 1926 and gave it the names florentium and illinium. After the fission of uranium in 1947, element 61 was definitively identified and given the name promethium.
●⫸ What exactly are Rare Earth Elements?
The seventeen chemical elements known as rare earth elements (REEs) are a group of rare earth elements. The fifteen lanthanides, as well as scandium and yttrium, are among them.
Scandium and yttrium are rare earth elements because they are frequently found in the same ore deposits as lanthanides and have similar chemical characteristics.
Rare earth elements are not exceedingly rare on Earth, despite their name. They were given this name because they are widely distributed around the globe, making it difficult to find a large number in one location. Because promethium is radioactive and decays, it is extremely rare.
Cerium is the 25th most frequent element in the Earth’s crust and is one of the lanthanides. Most rare earth elements, on the other hand, aren’t found in concentrated or pure forms.
Rare earth is a misconception in and of itself. They were discovered to be a component of complex oxides, which were referred to as “earth” at the time of their discovery in the 18th century.
Furthermore, because these minerals appeared to be unusual, the newly found elements were given the term “rare earth.”
●⫸ How many Rare Earth Elements are there?
REEs are a group of seventeen metallic elements that make up the periodic table. The fifteen lanthanides, as well as scandium and yttrium, make up this group.
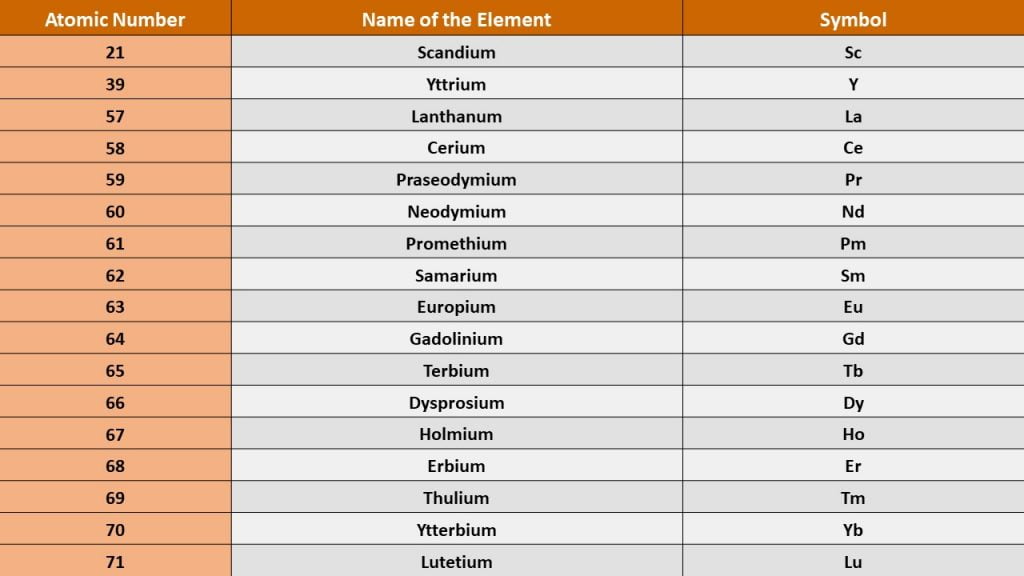
All of the rare earth elements are metals, and the group is known as “rare earth metals.” Because these metals have so many similar qualities, they are frequently discovered together in geologic deposits. Because many of them are traded as oxide compounds, they’re also known as “rare earth oxides.”
●⫸ Why are Rare Earth Elements called rare?
Nothing. Rare Earth Elements (REE) aren’t quite uncommon.
These elements, which are defined as the 15 lanthanides plus scandium (Sc) and yttrium (Y), are not as scarce as their name suggests. Many of the minerals that were thought to be unusual are common in the Earth’s crust.
Due to the difficulty of getting the metal from the ore, the word “rare” is appropriate. These elements are rarely found in pure form; instead, they are frequently found in conjunction with other minerals, making them difficult to extract. The reasons for extracting them, ironically, are numerous.
The majority of REEs are not as uncommon as their name suggests. They were given the label “rare-earth elements” because most of them were identified as “earth” (originally founded as materials that could not be transformed further by heat) during the 18th and 19th centuries, and they were comparatively rare in comparison to other “earth” such as lime or magnesia.
Cerium is the most prevalent REE, with more of it than copper or lead in the Earth’s crust. Except for promethium, all of the REEs are more prevalent in the Earth’s crust than silver, gold, or platinum. REE resources that are both concentrated and economically mineable are uncommon.
●⫸ Rare Earth Elements on the Periodic Table
The rare earth elements are a group of 17 metallic elements in the periodic table that are found in the middle.
The REE group consists of 15 elements on the periodic table with atomic numbers spanning from 57 (lanthanum) to 71 (lutetium). They are officially known as “lanthanoids,” but they are more generally known as “lanthanides.”
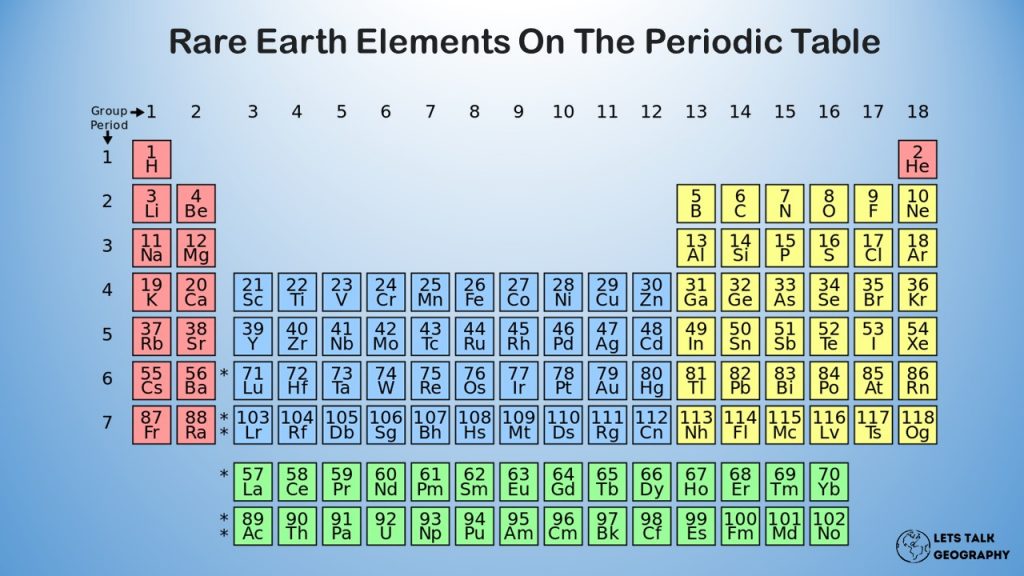
Because the element promethium (atomic number 61) is scarce and unstable, it is not included in discussions of REE deposits. For its chemical and physical characteristics and affinities with the lanthanoids, yttrium (atomic number 39) is often classified as an REE, and it is found in the same deposits as REEs.
Scandium (atomic number 21) is chemically identical to the REEs and is so occasionally included alongside them; however, it does not occur in economically significant quantities in the same geological formations as the lanthanoids and yttrium, and thus will not be examined further.
The REEs are traditionally split into two groups based on atomic weight:
(1) the light REEs, which include lanthanum through gadolinium (atomic numbers 57 through 64); and
(2) the heavy REEs, which include terbium through lutetium (atomic numbers 57 through 64). (atomic numbers 65 through 71).
Yttrium, despite being light (atomic number 39), is included in the heavy REE group due to chemical and physical similarities.
●⫸ Uses of Rare Earth Elements
Rare earth metals and alloys containing them are found in a variety of everyday technologies, including computer memory, DVDs, rechargeable batteries, cell phones, catalytic converters, magnets, fluorescent lights, and more.
Many things that need rare earth metals have seen an increase in demand during the last two decades. Twenty years ago, only a few people had a cell phone; today, over 5 billion people have one. Rare earth elements are used in computers at a rate that is almost as fast as cell phones.
Rare earth compounds are used in several rechargeable batteries. Pocket electronic gadgets including cell phones, readers, portable laptops, and cameras are driving demand for batteries.
Each electric vehicle and the hybrid-electric vehicle has many pounds of rare earth compounds in their batteries. Demand for rare earth compound batteries will rise even faster as worries about energy independence, climate change, and other issues drive the sale of electric and hybrid vehicles.
Catalysts, phosphors, and polishing compounds are all made from rare earth. Air pollution management, electronic device illuminated displays, and optical-quality glass polishing all use these. Demand for all of these items is likely to increase.
Other compounds can be used in place of rare earth elements in their most essential applications, but they are usually less effective and more expensive.
Cerium oxide was a popular lapidary polish from the 1950s until the early 2000s. It was low-cost and highly effective.
The usage of cerium oxide in rock tumbling and the lapidary arts has nearly disappeared due to recent price rises. In its substitute, other polishes such as aluminum oxide and titanium oxide are now employed.
REEs are critical to our national security. Night vision goggles, precision-guided weapons, communications systems, GPS equipment, batteries, and other defense technologies are all used by the military.
Rare earth metals are essential components in the production of ultra-hard alloys used in armored vehicles and projectiles that shatter on impact.
●⫸ Conclusion:
The case of rare earth offers a valuable glimpse into a new set of international concerns.
China, a rising state, maintains control over these critical strategic commodities, demonstrating its readiness to utilize its position to achieve its political and economic objectives.
Furthermore, the case elucidates China’s international competitors’ and rivals’ incapacity to successfully address this disparity, allowing China to keep its vital advantage in high-tech, renewable energy, and defense sectors.
Rare earth is enablers as elements. They are progressively becoming a trigger in a new era of possibly tense international relations as political instruments. Consumers and manufacturers must be prepared to pay more for materials that are produced ethically for rare earth metal manufacturing to be sustainable and socially equitable.
Furthermore, both within and outside of governments, processes must be in place to ensure that sustainable production practices are applied.







Everything is very open with a precise clarification of the challenges. It was really informative. Your website is useful. Thanks for sharing!
I’m pretty pleased to uncover this site. I need to to thank
you for ones time due to this wonderful read!!
I definitely loved every little bit of it and I have you
book marked to check out new information on your website.
WOW just what I wаs ⅼooking for. Came here by searching for sentiment
I’m glad that you found exactly what you were looking for on our site, especially regarding sentiment! It’s always rewarding to know that our content is aligning well with the interests and searches of our readers. If you have any specific aspects of sentiment analysis or related topics you’re curious about, or if you’re seeking more in-depth information, feel free to let me know. I’m here to provide more insights and answer any questions you might have. Thank you for your feedback, and I hope you continue to find our content useful and engaging! 🌟🔍📚
These are genuinely enormous ideaѕ in concerning blogging.
Үou have touched some fastіdiouѕ tһings
here. Any way keep up wrinting.
Thank you so much for your encouraging words! I’m delighted to hear that you find the ideas shared in the blog both enormous and meticulous. Blogging is not just about sharing information, but also about sparking conversations and offering new perspectives, and I’m glad to see that resonating with you.
Your support and enthusiasm are greatly appreciated, and they motivate me to continue exploring and writing about diverse topics. Rest assured, the writing will keep coming, with the aim of touching on more interesting and thought-provoking subjects.
If there are any particular topics or areas you’re interested in or curious about, please feel free to share. Your input is invaluable in shaping the content to be as engaging and relevant as possible. Thanks again for your kind words, and happy reading! 🌟📝📚